Prepared by Julie Kim, M.D., F.A.C.S., F.A.S.M.B.S., Ann Rogers, M.D., Dan Eisenberg, M.D., Guilherme M. Campos, M.D., Dan Azagury, M.D.
The following position statement has been issued by the American Society for Metabolic and Bariatric Surgery in response to numerous inquiries made to the Society by patients, physicians, society members, hospitals, health insurance payors, the media, and others regarding the benefit of metabolic and bariatric surgery on long-term survival. An overview of the current available published peer-reviewed scientific evidence is presented. (Surg Obes Relat Dis 2016;12:453–459.) © 2016 American Society for Metabolic and Bariatric Surgery. All rights reserved.
Rates of obesity continue to increase worldwide. Obesity-related co-morbid conditions are associated with reduced quality of life and life expectancy. Certain obesity-related co-morbid conditions (cardiovascular disease [CVD], can-
cer, and type 2 diabetes [T2D]) are the leading causes of death in the world [1]. Metabolic and bariatric surgery remains the most effective treatment for most patients with clinically severe obesity (class II and class III obesity), with a safety profile better than many commonly performed general surgery procedures [2]. Current metabolic bariatric operations (sleeve gastrectomy, Roux-en-Y gastric bypass, and duodenal switch) result in significant and durable weight loss as well as metabolic changes independent of weight loss. These changes, which can occur rapidly, have been shown to be associated with high rates of T2D remission. Metabolic and bariatric surgery has been shown to be associated with reduced CVD risk factors; reduced CVD events; and reduced deaths related to selected cancers, T2D, and CVD events. Data suggest that metabolic and bariatric surgery, in addition to reducing all-cause mortality, may improve survival and longevity. In light of these findings and the unmet need of obesity treatment, ongoing efforts to reduce barriers to access to metabolic and bariatric surgery are necessary.
Obesity and mortality
According to a recent estimate from the World Health Organization, over 600 million people, or 13% of all adults worldwide, are obese, with a body mass index (BMI) ≥30 kg/m2. In addition, most people in the world live in countries where being overweight and obese are more often linked to death than being underweight [3]. Obesity is an established major risk factor for heart disease, stroke, T2D, some cancers, and other associated medical problems that can increase an individual’s risk of early death. The risk for such diseases increases in parallel with increasing BMI. Similarly, obesity in childhood and adolescence tends to predict adult obesity and elevated mortality risk [4].
Although there is conflicting evidence on the risks of overweight versus class I obesity (BMI 30–34.9 kg/m2 causing premature death, data from several large and well-conducted case control studies indicate that individuals with class II (BMI 35–39.9 kg/m2 m2) and class III (BMI ≥40 kg/m2) obesity have significantly higher all-cause mortality rates than leaner individuals. In addition, patients with BMIs that qualify for bariatric surgery but do not undergo the procedure due to personal preference or insurance denials have higher mortality rates compared with cohorts who did undergo bariatric surgery [5,6]. A recent systematic review and meta-analysis of 97 articles reported on hazard ratios (HR) for all-cause mortality in relation to BMI categories and showed that, relative to people of normal weight, people with obesity of all classes have higher all-cause mortality [7]. This was not shown to be the case when looking specifically at class I obesity, which was associated with lower all-cause mortality.
A study using National Health and Nutrition Examination Survey data found that, compared with adults of normal weight, adults with class II and III obesity died 3.7 years earlier (all-cause mortality), and adults with class III obesity had CVD-related deaths 5 years earlier [8]. Similarly, a pooled analysis of 20 prospective studies from developed countries found that class III obesity was associated with a substantially increased risk of all-cause mortality compared with individuals of normal weight, with most of the deaths due to CVD, cancer, and diabetes [9].
Life expectancy in patients with obesity has also been studied in the context of BMI “trajectories.” A study using data from the U.S. Health and Retirement Study, which included adults aged 51 to 77 years, defined 6 categories of BMI trajectories. These included normal weight downward, normal weight upward, overweight stable, overweight obesity, class I obese upward, and class II/III obese upward. Confirming a finding seen in prior studies, individuals in the overweight stable group had the highest survival rate, while individuals in the class II/III upward trajectory had the lowest survival rate. This finding persisted after controlling for socioeconomic factors and multiple chronic illness states. Using an attributable risk analysis, the authors found that about 7.2% of deaths after age 51 were due to obese upward trajectories [10].
To differentiate the specific effect of obesity from that of obesity-related co-morbid conditions or metabolic health on mortality risk, data from the Whitehall II cohort, a longitudinal study of 10,308 United Kingdom government employees was examined. BMI and metabolic factors were available for 5269 individuals. A total of 638 individuals (12.1% of the cohort) were identified as obese, of whom 9% to 41% were identified as “metabolically healthy obese patients” depending on the definition of metabolic health used (Matsuda, homeostatic model assessment [HOMA] index, adult treatment panel III [ATP-III], Karelis and Wildman criteria). Interestingly, regardless of the definition of metabolic health used, with the exception of the HOMA index definition, all “metabolically healthy obese patients” still had an increased risk of mortality and an increased risk of cardiovascular mortality compared with individuals of normal weight [11].
Therefore, it is reasonable to conclude that obesity in general, and clinically severe obesity in particular, can shorten life expectancy, even in the patients who are considered to be “metabolically healthy.” As stated earlier, obesity is a well-known independent risk factor for the development of CVD, T2D, and some cancers, and these conditions are the leading causes of mortality in patients with obesity ][11].
Cardiovascular disease
There is a large body of observational data to support that metabolic and bariatric surgery is associated with improvement in CVD risk factors. A systematic review and meta-analysis by Gloy et al. attempted to quantify the overall effects of bariatric surgery compared with nonsurgical treatment for obesity, in particular looking at risk factors for CVD, and included 11 randomized trials with 796 patients. A significant reduction in weight and plasma LDL levels, an increase in plasma HDL levels, and increasing remission rates in T2D were identified after metabolic and bariatric surgery [12]. Another recent systematic review and meta-analysis by Vest et al. looked at 73 different CVD risk factors after metabolic and bariatric surgery, including 18 studies with 19,453 participants. They found a significant reduction in weight, hypertension, T2D, hyperlipidemia, and adverse echocardiographic parameters after metabolic and bariatric surgery [13]. Neither of these systematic reviews, however, was able to report the effect of metabolic and bariatric surgery and future CVD events.
A recent meta-analysis by Kwok et al. included 14 studies with 29,208 surgical patients and 166,200 non-
surgical controls, with a follow-up of 2–14.7 years [14]. It was the first study to show that bariatric surgery is associated with a reduced risk of other CVD events, including myocardial infarction and stroke, and composite adverse CVD events compared with nonoperated cohorts (50% risk reduction in the surgical group). In a pooled analysis of 4 studies with adjusted data, metabolic and bariatric surgery was associated with a significantly reduced risk of composite CVD adverse events, myocardial infarction, and stroke. Randomized clinical trials may be needed to validate the observational findings of decreased CVD risk, decreased likelihood of future CVD events, and CVD mortality after metabolic and bariatric surgery; however, the feasibility of such randomized clinical trials may be impractical given the large numbers of both patients and years of follow-up that would be required, as well as ethical concerns of withholding surgical treatment.
Diabetes
Estimates from the Centers for Disease Control 2009–2012 National Health and Nutrition Examination Survey estimates applied to 2012 U.S. census data, show that 29.1 million people, or 9.3% of the U.S. population, have diabetes, and T2D accounts for approximately 95% of all diagnosed cases of diabetes [15]. Most patients with T2D are obese, and patients with T2D who are not obese by traditional weight or BMI criteria generally have an increased percentage of body fat distributed predominantly in the abdominal region [16].
Diabetes is associated with increased risk for multiple other end-organ damage leading to an increased incidence of CVD, heart failure, and stroke; it is also the leading cause of kidney failure, lower-limb amputation, and blindness. Hospitalization rates for myocardial infarction, stroke, and CVD-related death rates have been shown to be about 1.7, 1.5, and 1.8 times higher, respectively, among adults aged 18 years or older with T2D than among adults without diagnosed diabetes. T2D is likely underreported as a cause of death. Previous reports estimated that as many as 35% to 40% of people with T2D who die did not have T2D listed on the death certificate, and only about 10% to 15% had it listed as the cause of death [15].
Metabolic and bariatric surgery has profound effects on glycemic control in the general population. It is associated with initial remission of T2D in as many as 68% of patients, with complete and long-term remission in approximately 33% of patients and significant reduction in the doses of antidiabetic medications needed to maintain euglycemia [17]. Data from 5 randomized controlled trials have shown superior T2D control in patients who underwent metabolic and bariatric surgery versus conventional medical management [18–22]. Multiple controlled and cohort studies of metabolic and bariatric surgery have shown significant remission rates and prevention of T2D and also improvement or complete remission of other CVD, pulmonary, renal, and vascular diseases associated with obesity and T2D [23]. In addition, 5 studies with long-term follow-up reported a reduction in death rates related to improvement in T2D, CVD, and cancer [2,24,25].
Recently, Sjöström et al. reported that in patients with obesity and T2D from the Swedish Obese Subjects (SOS) trial, a 2-cohort prospective observational study with long-term follow-up, metabolic and bariatric surgery was more frequently associated with T2D remission and fewer micro and macrovascular complications than usual nonsurgical care [26]. These findings were identified despite most patients having undergone purely restrictive procedures such as the vertical banded gastroplasty, which have not been shown to have weight independent metabolic changes.
Although all available evidence shows that metabolic and bariatric surgery is the most effective treatment for patients with obesity and T2D alone, randomized data showing decreased mortality rates due to conditions associated with T2D are not available. In addition, no study has solely evaluated whether metabolic and bariatric surgery can reduce mortality rates in patients with T2D.
A recent study used a Markov model with logistic regression models as inputs to compare metabolic and bariatric surgery versus no surgical treatment for patients with severe obesity and T2D. Data were collected from 3 large separate cohorts: the HMO Research Network sites, the Nationwide Inpatient Sample, and the National Health Interview Survey linked to the National Death Index. Their analyses found that a 45-year-old woman with T2D and a BMI of 45 kg/m2 gained an additional 6.7 years of life expectancy with metabolic and bariatric surgery (38.4 years with versus 31.7 years without surgery), but sensitivity analyses revealed that the gain in life expectancy decreased with increasing BMI, up to a BMI of 62 kg/m2 point nonsurgical treatment was associated with greater life expectancy [27]. These findings should be interpreted with caution, as an earlier study published by the same authors, though not controlling for T2D, reported that higher BMIs were associated with larger increases in life expectancy gains after metabolic and bariatric surgery [28]. Limitations of the more recent study may include an overestimation of 30-day mortality in surgery patients (as compared with current mortality rates), lack of quality of life data, and the fact that only 2.7% of surgery patients had a BMI of 60 kg/m2 or more [19]. Level I and II evidence supports the hypothesis that metabolic and bariatric surgery is significantly superior to nonsurgical management for the treatment for T2D and that mortality associated with T2D is reduced after surgery. Ongoing research is recommended to adequately study the impact of metabolic and bariatric surgery on life expectancy in patients with T2D.
Cancer
Like obesity, the incidence of cancer has also increased throughout the world and is expected to increase 57% in the next 20 years [29]. Although cancer is a disease of mutated genes, the minority of all cancers are familial or inherited. It is understood that external factors contribute to the risk of developing cancer. To what extent a contributing factor may be ameliorated or altered may affect its magnitude of contribution. According to the World Health Organization, tobacco use, alcohol use, unhealthy diet, and physical inactivity are the main cancer risk factors worldwide, and > 30% of cancer deaths could be prevented by modifying or avoiding key risk factors [29].
According to the American Cancer Society, the link between obesity and cancer is strongest for breast cancer in women past menopause; colon and rectal cancers; and cancers of the esophagus, kidney, pancreas, and endometrium. The hormonal changes promoting the production of peptides and steroid hormones as well as the inflammatory dysregulation and insulin resistance mediated by adipose tissue are some of the leading contributing factors linking obesity to cancer [30].
Increased rates of cancer have been published for individuals with obesity ranging from 2.1–5.8 cases per 1000 person-years. One of the first large observational studies to demonstrate cancer risk reduction in individuals with morbid obesity undergoing bariatric surgery was the McGill Bariatric Cohort Study, which involved 1035 individuals who underwent metabolic and bariatric surgery with 5746 individuals that did not undergo surgery. The patients were followed for 5 years. Reduced cancer rates of 2.03% were found in the surgery group compared with 8.49% (relative risk = .24, 95% CI = .17–.39) in the nonsurgical cohort. An overall reduction in mortality in the surgical group was also identified. The mortality rate in the surgical group was .68% compared with 6.17% for the nonsurgical cohort (relative risk = .11, 95% CI = .04–.27) [31,32]. The specific reduction in mortality from cancer, however, was not defined.
A recent systematic review and meta-analysis looking at the frequency of cancer in patients following metabolic and bariatric surgery, included 13 studies (4 cohort and 9 uncontrolled) with 11,087 patients who underwent surgery and 20,720 patients in the nonsurgical group and identified a reduced cancer risk in obese individuals undergoing metabolic and bariatric surgery with a cancer incidence density rate of 1.06 cases per 1000 person-years [33]. Another meta-analysis of 4 observational studies with 24,321 individuals with obesity who underwent metabolic and bariatric surgery and 80,806 individuals with obesity who did not undergo surgery, looked specifically at the effects of metabolic and bariatric surgery on colorectal cancer, where obesity is an established risk factor, and showed a 27% risk reduction [34].
Results published in 2007 from the SOS intervention study, which included 2010 patients with obesity who underwent metabolic and bariatric surgery (surgical group) and 2037 patients with obesity who did not undergo surgery (cohort group), revealed a strong effect of cancer on mortality in patients with obesity, as cancer was found to be the most common cause of death in the study with 47 cancer deaths in the cohort group and 29 cancer deaths in the surgical group (the second most common cause of death being myocardial infarction) [35]. More recently, Sjöström published findings from the longitudinal SOS trial showing that metabolic and bariatric surgery was associated with a significant reduction of cancer in women, with 79 first-time cancers in the surgical group compared with 130 first-time cancers in the control, cohort group (unadjusted HR = .58; 95% CI .44–.77; P = .0001). Although the rate was reduced in men, the reduction was not statistically significant [24].
Similar findings were also published in a meta-analysis by Tee et al., which looked at studies comparing the relative risk of cancer in obese patients undergoing metabolic and bariatric surgery versus obese control patients, with protective benefits of bariatric surgery seen in women with reduced cancer risk and cancer mortality, which was not statistically significant in men [36]. The lack of randomized controlled data limits the ability to validate whether a true gender benefit is present as well as the true impact of metabolic and bariatric surgery on cancer risk and cancer outcomes. Nevertheless, metabolic and bariatric surgery is associated with a decreased incidence of some cancers and a decreased cancer-related mortality in women.
Survival
Multiple studies have evaluated long-term mortality rates of patients with clinically severe obesity after metabolic and bariatric surgery relative to patients who did not undergo weight loss surgery (Table 1) [2,32,35,37–44]. An early study examining the mortality and healthcare use of patients with clinically severe obesity conducted by Christou et al. compared 5-year outcomes of patients who had metabolic and bariatric surgery at a single institution with an age and sex-matched cohort using a health insurance database. They found a significant relative risk reduction of death of 89% after surgery. Other than age and sex, however, there was no information provided regarding other co-morbid conditions and how they compared between cohorts [32].
At the same time, an observational study, with follow-up data after 15 years, comparing metabolic and bariatric surgery and nonoperative age, sex, and Charlson index-matched patients, using hospital discharge data from the Washington State Comprehensive Hospital Abstract Reporting System database, reported improved survival (HR = 1.59; 95% CI, 1.49–1.72) in the surgical cohort [37]. Zheng et al. sought to quantify the long-term rate of mortality in patients undergoing metabolic and bariatric surgery. They used the International Bariatric Surgery Registry to identify an all-cause mortality rate of 3.45%, after a mean of 8.3 years in nearly 19,000 patients [10].
In an effort to compare the long-term mortality of metabolic and bariatric surgery, surgical patients were compared with a matched cohort of nonsurgical patients with self-reported BMIs. Adams et al. studied 7925 bariatric surgical patients and 7925 patients in the general population with a mean follow up of 7.1 years. The authors found the adjusted long-term all-cause mortality decreased by 40% in the surgical group compared with the control group. Cause-specific mortality decreased for obesity-related co-morbid conditions, such as CVD, T2D, and cancers, but the rates of death from causes not related to disease, such as accidents and suicides, increased in the surgical group [41].
Of the studies examining mortality after metabolic and bariatric surgery, the study by Maciejewski et al. focused on the high-risk bariatric population receiving treatment in the Veterans Affairs (VA) hospital system. These patients are largely male, have a higher BMI, and a greater proportion have obesity-related co-morbid conditions. Similar to other studies, the authors found that crude mortality rates were lower in the surgery cohort compared with nonsurgical obese controls: at 1 year, 1.5% versus 2.2%, P = .17; at 2 years, 2.2% versus 4.6%, P < .001; at 6 years, 6.8% versus 15.2%, P < .001. However, in a subset of patients who were propensity score-matched to controls, there was no significant difference in mortality between the groups at a mean follow-up of 6.7 years [42]. In a follow-up to this study, Arterburn et al. re-examined long-term mortality in the VA cohort with a longer follow-up period and a larger sample size of patients. Using the Veterans Affairs Surgical Quality Improvement Program database, 2500 veteran patients were age, BMI, and Diagnostic Cost Group–matched to nonoperative veteran cohorts. Most of the surgical patients were male, white, and had T2D. With a 14-year study period and a mean follow-up of 6.9 years, survival benefit was seen in the surgical patients at 5 years and 10 years after metabolic and bariatric surgery. A multivariable-adjusted Cox regression found that metabolic and bariatric surgery was associated with lower mortality after 1 to 5 years (HR = .45; 95% CI .36–.56) and 5 or more years of follow-up (HR = .47 95% CI, .39–.58), but not associated with lower mortality in the first year after surgery [2].
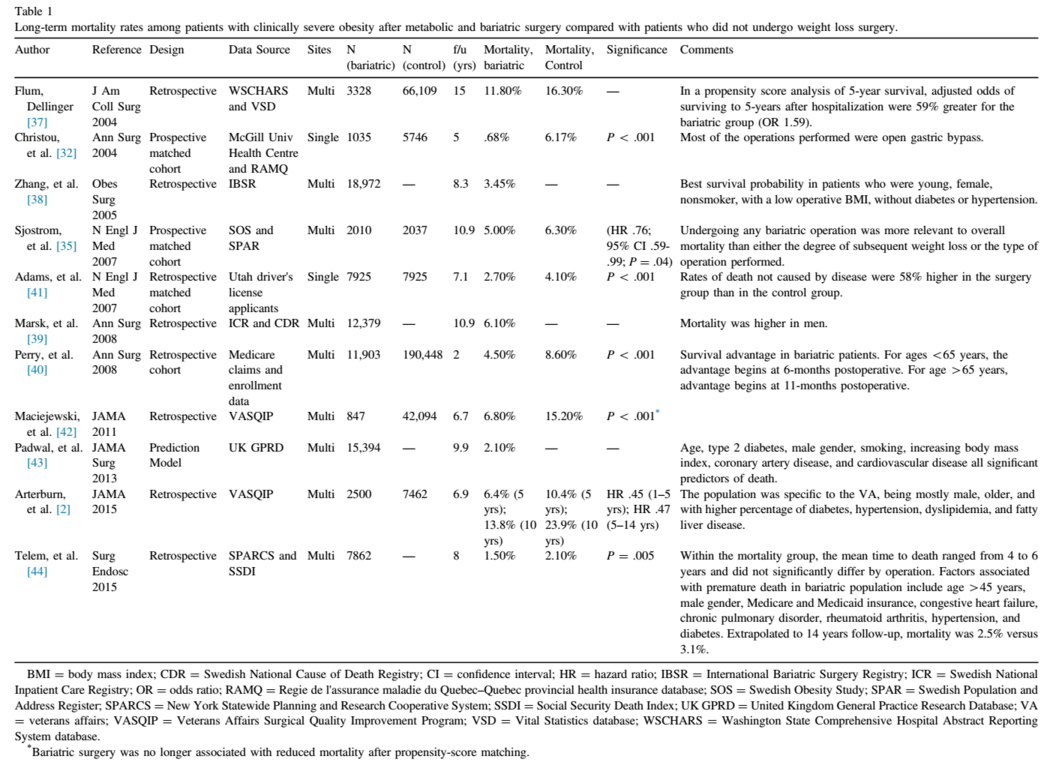
A retrospective study using the Swedish National Inpatient Care Registry to review all bariatric surgery performed between 1980 and 2005 found a lower rate of all-cause mortality among patients undergoing metabolic and bariatric surgery. Their results mirrored those of the SOS Study. The SOS is unique in its design as a prospective, controlled, cohort study, and was planned to specifically address the issue of survival improvement after metabolic and bariatric surgery. Cohorts of 42000 patients were followed for a mean of 11 years and matched to nonsurgical patients according to 18 clinical variables and were found to have a significantly lower mortality, 5.0% versus 6.3%. Differences between the cohorts were most notable beyond 8 years after metabolic and bariatric surgery. However, 84% of the patients in this study underwent a vertical banded gastroplasty or insertion of a gastric band, operations without metabolic changes that have since been mostly abandoned, thus potentially limiting the relevance of the findings [24].
Also in Europe, Padwal et al. used a population-representative primary care registry to construct a clinical prediction model for 10-year all-cause mortality for patients who met 1991 National Institutes of Health criteria for metabolic and bariatric surgery, by linking with the mortality records of the United Kingdom Office of National Statistics. The findings of an all-cause mortality rate of 2.1% was lower than most patient-based studies, with age, T2D, male sex, smoking, increasing BMI, and CVD all significant predictors of death; however, based on their model, of the obesity-related co-morbid conditions, T2D was the most important mortality predictor [43].
A recent study comparing metabolic and bariatric surgical patients to the general (nonsurgical) population confirmed a significant improvement in long-term survival in the surgical cohort. In a study of 7862 laparoscopic metabolic and bariatric surgery patients using longitudinal administrative data of the New York State Planning and Research Cooperative System compared with actuarial projections of New York State mortality rates from the Centers of Disease Control, Telem et al. found a mortality rate of 1.5% versus 2.1% (P = .005). Extended from 8 to 14 years, the surgery patients’ observed mortality was 2.5%, compared with an extrapolated actuarial mortality rate predicted as 3.1% (P = .01) [44].
Patients undergoing metabolic and bariatric surgery have decreased long-term mortality relative to weight-matched controls. Studies using multiple patient settings as well as nonpatient databases confirm similar results of significantly improved long-term survival after metabolic and bariatric surgery; a benefit to surgery that may begin as early as 2 years postoperatively.
Findings relative to the nonsurgical usual care treatment of individuals with class II and III obesity are summarized in the following:
-
Metabolic and bariatric surgery is associated with a reduction in all-cause mortality and increased long-term survival.
-
Metabolic and bariatric surgery is significantly superior to current best medical management for the treatment and remission of T2D and reduces death rates associated with T2D.
-
Metabolic and bariatric surgery is associated with a reduction of CVD risk factors and future CVD events and deaths.
-
Metabolic and bariatric surgery is associated with a reduction in the risk of certain cancers and improved cancer outcomes and mortality for women.
References
[1] Yoon PW, Bastian B, Anderson RN, Collins JL, Jaffe HW. Centers for Disease Control and Prevention (CDC). Potentially preventable deaths from the five leading causes of death—United States, 2008– 2010. MMWR Morb Mortal Wkly Rep 2014;63(17):369–74.
[2] Arterburn DE, Olsen MK, Smith VA, et al. Association between bariatric surgery and long-term survival. JAMA 2015;313(1):62–70.
[3] World Health Organization. Obesity and Overweight [homepage on the Internet]. Fact Sheet No. 311 [cited 2015 Nov 16]. Available from: http://www.who.int/mediacentre/factsheets/fs311/en/.
[4] Engeland A, Bjørge T, Tverdal A, Søgaard AJ. Obesity in adolescence and adulthood and the risk of adult mortality. Epidemiology 2004;15(1):79–85.
[5] MacDonald KG Jr, Long SD, Swanson MS, et al. The gastric bypass operation reduces the progression and mortality of non-insulin-dependent diabetes mellitus. J Gastrointest Surg 1997;1 (3):213–20.
[6] Sowemimo OA, Yood SM, Courtney J, et al. Natural history of morbid obesity without surgical intervention. Surg Obes Relat Dis 2007;3(1):73–7.
[7] Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA 2013;309(1):71–82.
[8] Borrell LN, Samuel L. Body mass index categories and mortality risk in US adults: the effect of overweight and obesity on advancing death. Am J Public Health 2014;104(3):512–9.
[9] Kitahara CM, Flint AJ, Berrington de, Gonzalez A, et al. Association between class III obesity (BMI of 40–59 kg/m2) and mortality: a pooled analysis of 20 prospective studies. PLoS Med 2014;11 (7):e1001673.
[10] Zheng H, Tumin D, Qian Z. Obesity and mortality risk: new findings from body mass index trajectories. Am J Epidemiol 2013;178 (11):1591–9.
[11] Danaei G, Ding EL, Mozaffarian D, et al. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med 2009;6(4):e1000058.
[12] Gloy VL, Briel M, Bhatt DL, et al. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ 2013;347:f5934.
[13] Vest AR, Heneghan HM, Agarwal S, Schauer PR, Young JB. Bariatric surgery and cardiovascular outcomes: a systematic review. Heart 2012;98(24):1763–77.
[14] Kwok CS, Pradhan A, Khan MA, et al. Bariatric surgery and its impact on cardiovascular disease and mortality: a systematic review and meta-analysis. Int J Cardiol 2014;173(1):20–8.
[15] Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta, GA: U.S. Department of Health and Human Services; 2014.
[16] American Diabetes Association. The American Diabetes Association (ADA) has been actively involved in the development and dissemination of diabetes care standards, guidelines, and related documents for many years. Introduction. Diabetes Care 2009;32(Suppl 1):S1–2.
[17] Arterburn DE, Bogart A, Sherwood NE, et al. A multisite study of long-term remission and relapse of type 2 diabetes mellitus following gastric bypass. Obes Surg 2013;23(1):93–102.
[18] Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med 2012;366(17):1577–85.
[19] Schauer PR, Bhatt DL, Kashyap SR. Bariatric surgery versus intensive medical therapy for diabetes. N Engl J Med 2014;371 (7):682.
[20] Courcoulas AP, Goodpaster BH, Eagleton JK, et al. Surgical vs medical treatments for type 2 diabetes mellitus: a randomized clinical trial. JAMA Surg 2014;149(7):707–15.
[21] Dixon JB, O’Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA 2008;299(3):316–23.
[22] Ikramuddin S, Korner J, Lee WJ, et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA 2013;309(21):2240–9.
[23] Yu H, Di J, Bao Y, et al. Visceral fat area as a new predictor of short-term diabetes remission after Roux-en-Y gastric bypass surgery in Chinese patients with a body mass index less than 35 kg/m2 Obes Relat Dis 2015;11(1):6–11.
[24] Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) trial—a prospective controlled intervention study of bariatric surgery. J Intern Med 2013;273(3):219–34.
[25] Adams TD, Stroup AM, Gress RE, et al. Cancer incidence and mortality after gastric bypass surgery. Obesity (Silver Spring) 2009;17(4):796–802.
[26] Sjöström L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 2014;311 (22):2297–304.
[27] Schauer DP, Arterburn DE, Livingston EH, et al. Impact of bariatric surgery on life expectancy in severely obese patients with diabetes: a decision analysis. Ann Surg 2015;261(5):914–9.
[28] Schauer DP, Arterburn DE, Livingston EH, Fischer D, Eckman MH. Decision modeling to estimate the impact of gastric bypass surgery on life expectancy for the treatment of morbid obesity. Arch Surg 2010;145(1):57–62.
[29] Stewart BW, Wild CP. World cancer report 2014. Lyon, France: International Agency for Research on Cancer. WHO Press; 2014.
[30] Ashrafian H, Ahmed K, Rowland SP, et al. Metabolic surgery and cancer: protective effects of bariatric procedures. Cancer 2011;117 (9):1788–99.
[31] Christou NV. Impact of obesity and bariatric surgery on survival. World J Surg 2009;33(10):2022–7.
[32] Christou NV, Sampalis JS, Liberman M, et al. Surgery decreases long-term mortality, morbidity, and health care use in morbidly obese patients. Ann Surg 2004;240(3):416–23.
[33] Casagrande DS, Rosa DD, Umpierre D, Sarmento RA, Rodrigues CG, Schaan BD. Incidence of cancer following bariatric surgery: systematic review and meta-analysis. Obes Surg 2014;24(9):1499–509.
[34] Afshar S, Kelly SB, Seymour K, Lara J, Woodcock S, Mathers JC. The effects of bariatric surgery on colorectal cancer risk: systematic review and meta-analysis. Obes Surg 2014;24(10):1793–9.
[35] Sjöström L, Narbro K, Sjöström CD, et al. Swedish Obese Subjects Study. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007;357(8):741–52.
[36] Tee MC, Cao Y, Warnock GL, Hu FB, Chavarro JE. Effect of bariatric surgery on oncologic outcomes: a systematic review and meta-analysis. Surg Endosc 2013;27(12):4449–56.
[37] Flum DR, Dellinger EP. Impact of gastric bypass operation on survival: a population-based analysis. J Am Coll Surg 2004;199 (4):543–51.
[38] Zhang W, Mason EE, Renquist KE, Zimmerman MB, IBSR Contributors. Factors influencing survival following surgical treat-
ment of obesity. Obes Surg 2005;15(1):43–50.
[39] Marsk R, Freedman J, Tynelius P, Rasmussen F, Näslund E. Antiobesity surgery in Sweden from 1980 to 2005: a population-
based study with a focus on mortality. Ann Surg 2008;248(1):777–81.
[40] Perry CD, Hutter MM, Smith DB, Newhouse JP, McNeil BJ. Survival and changes in comorbidities after bariatric surgery. Ann Surg
2008;247(1):21–7.
[41] Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med 2007;357(8):753–61.
[42] Maciejewski ML, Livingston EH, Smith VA, et al. Survival among high-risk patients after bariatric surgery. JAMA 2011;305(23):2419–26.
[43] Padwal RS, Klarenbach SW, Wang X, et al. A simple prediction rule for all-cause mortality in a cohort eligible for bariatric surgery. JAMA Surg 2013;148(12):1109–15.
[44] Telem DA, Talamini M, Laurie Shroyer A, et al. Long-term mortality rates (48-year) improve as compared to the general and obese population following bariatric surgery. Surg Endosc 2015;29 (3):529–36.